John Dalton
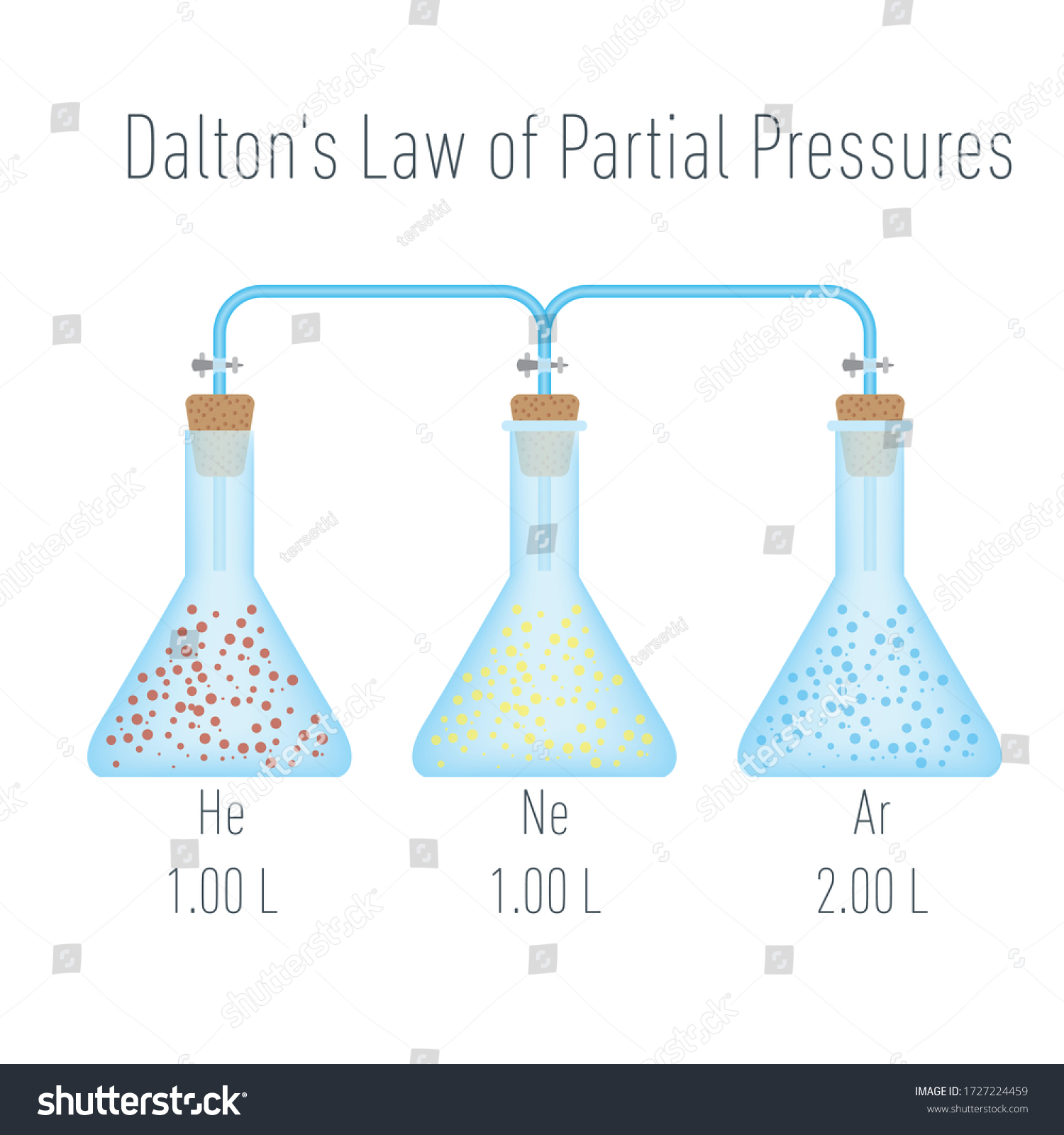
John Dalton revealed the concept of his law "Dalton's Law of Partial Pressures" which states All matter in terms of atoms and their properties expressing that each element had its own atom. And within the atoms laid various elements. This led him to have a closer way of examination in gasses and have the ability to determine the "ideal gasses" opposed to real gasses and this was due to low particle volume or elasticity within the gasses. Due to this law being established it helped assist various problems that may occur. In which with this law you're
able to calculate the molecules of different individual gasses.
able to calculate the molecules of different individual gasses.

Comments
Post a Comment